Automated de novo phasing and model building of coiled-coil proteins
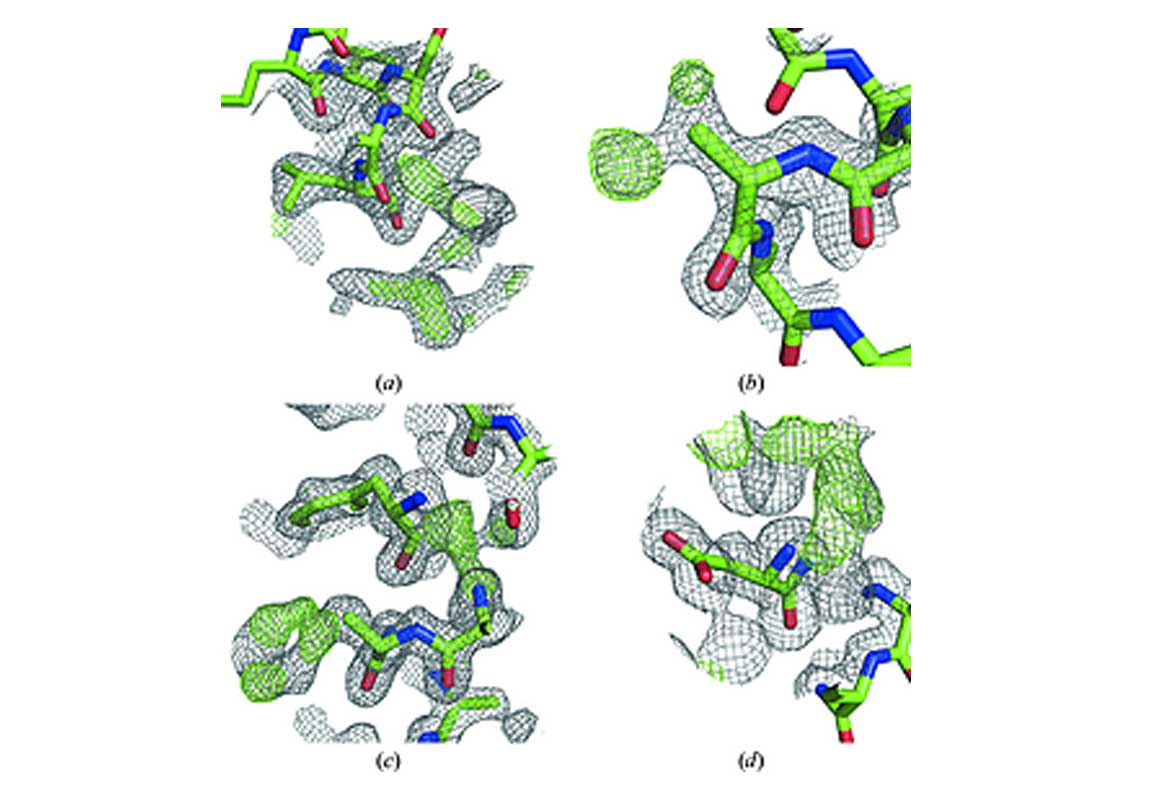
Models generated by de novo structure prediction can be very useful starting points for molecular replacement for systems where suitable structural homologues cannot be readily identified. Protein-protein complexes and de novo-designed proteins are examples of systems that can be challenging to phase. In this study, the potential of de novo models of protein complexes for use as starting points for molecular replacement is investigated. The approach is demonstrated using homomeric coiled-coil proteins, which are excellent model systems for oligomeric systems. Despite the stereotypical fold of coiled coils, initial phase estimation can be difficult and many structures have to be solved with experimental phasing. A method was developed for automatic structure determination of homomeric coiled coils from X-ray diffraction data. In a benchmark set of 24 coiled coils, ranging from dimers to pentamers with resolutions down to 2.5 Å, 22 systems were automatically solved, 11 of which had previously been solved by experimental phasing. The generated models contained 71-103% of the residues present in the deposited structures, had the correct sequence and had free R values that deviated on average by 0.01 from those of the respective reference structures. The electron-density maps were of sufficient quality that only minor manual editing was necessary to produce final structures. The method, named CCsolve, combines methods for de novo structure prediction, initial phase estimation and automated model building into one pipeline. CCsolve is robust against errors in the initial models and can readily be modified to make use of alternative crystallographic software. The results demonstrate the feasibility of de novo phasing of protein-protein complexes, an approach that could also be employed for other small systems beyond coiled coils.
